I am thrilled to share that after several years of research and development, our team has published the first of several manuscripts outlining some of the key principles and component technologies that we believe will completely revolutionize the practice of fat grafting and adipose-derived cellular therapeutics.
Adipose tissue, or fat, is the largest reservoir of adult stem cells in the body. These cells will soon be the workhorse in the space of regenerative medicine. For instance, they are being used to heal heart tissue after heart attack, restore nerves after spinal cord injury, and repair knees in need of replacement. Unfortunately, the traditional method of isolating these cells involves the use of dissociation enzymes which is impractical for widespread adoption because it requires special laboratory setup and dedicated professional staff.
Adipose tissue, or fat, is the largest reservoir of adult stem cells in the body. These cells will soon be the workhorse in the space of regenerative medicine.
In 2013, Dr. Tonnard and others first described ‘nanofat’ processing and grafting, in which fat tissue is manually and mechanically emulsified by repeatedly passing liposuction fat between two syringes, followed by filtration, to yield a therapeutic that was then injected to correct unsightly skin wrinkles and discoloration. In 2016, my co-authors and I took this analysis a step further by examining the rheologic properties of nanofat processing as well as a detailed analysis of its cellular components. This endeavor allowed us to identify the type of flow (laminar) and shear stress that results in the mechanical breakdown of fat tissue. Perhaps most exciting about our paper was that we identified several stem cell populations enriched by this technique, essentially identifying nanofat processing as a form mechanical stem cell isolation that obviates the need for special chemicals or technicians for point-of-care therapeutic applications.
This month (January 2023) myself and Drs. Jeremy Lombardo, PhD, Jered Haun, and Alan D Widgerow MBBCh(MD) MMed(MHS) FCS(Plast) FACS, published “Fluidic Device System for Mechanical Processing and Filtering of Human Lipoaspirate Enhances Recovery of Mesenchymal Stem Cells,” in the field’s largest journal, Plastic & Reconstructive Surgery. The work described in this article was supported by grants from the Plastic Surgery Foundation and the Institute for Clinical & Translational Science at UC Irvine.
In this study, we developed and tested novel fluidic devices that could be combined and automated to produce a nanofat-type injectable therapeutic. The Emulsification & Micronization Device (EMD) was designed to optimally leverage fluid shear forces to break down tissue and turbulent fluid mixing to emulsify lipid droplets. The Filtration Device (FD) was designed to filter out large, unwanted fragments while maximizing cellular recovery. Because this platform is automated, it increases the overall speed of processing fat tissue while reducing the inherent variability introduced by human operators. We used similar methods to our 2016 paper to analyze the viability and cellular makeup of the mechanically generated stromal vascular fraction (SVF) therapeutic.
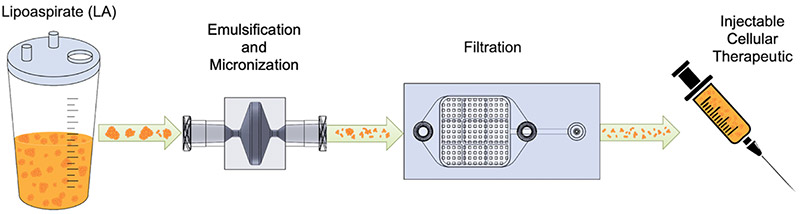
We are thrilled to share that our patent-pending platform, which is the foundation of my new medical device startup Sayenza Biosciences, produces a superior adipose cellular therapeutic when compared to traditional nanofat. This powerful regenerative injectable, which we have dubbed NESVF™ (non-enzymatic stromal vascular fraction), has similar cellular viability to nanofat, but with a >1.5 fold increase in cell percentages and a 2- to 3-fold increase in cells recovered per milliliter of fat processed. Notably, after enzymatic digestion, we observed significant enrichment in critical stem cell populations including mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs) and a subtype of MSCs critical for healing in diabetic wounds (DPP4+/CD55+). These findings were consistent for healthy fat processed by our platform, as well as diabetic fat, paving the way for our platform to have tremendous potential in the setting of difficult-to-treat diabetic wounds.
This is just the beginning. 2023 is poised to be a pivotal year for Sayenza Biosciences. Our second manuscript, which dives deeper into the cellular and molecular components of NESVF™, as well as its mechanisms of action essential to regenerative healing, is nearly ready for submission. I have been invited to serve as faculty at the 3rd Multispecialty Regenerative Surgery Course in Ghent, Belgium, sharing the stage among the world’s foremost thought leaders in regenerative surgery. And Sayenza Bio will grow as a company. We will be announcing some exciting new research findings, adding innovative and peerless talent to our team, and making strides to democratize regenerative medicine using fat, the body’s largest source of stem cells.
Published by: Derek Banyard, MD, MS, MBA